The pharmaceutical industry operates in a highly regulated environment where product quality, safety, and efficacy must remain consistent throughout a product’s lifecycle. As companies move products from research laboratories to commercial manufacturing, or from one manufacturing site to another, maintaining this consistency becomes a critical challenge. This is where Technology transfer in pharma plays a central role.
Technology transfer in pharma is a systematic, documented, and science-based process that ensures all relevant product and process knowledge is successfully transferred between development and manufacturing units. Whether it involves scaling up a formulation, transferring production to a new site, or outsourcing manufacturing to a contract organization, technology transfer is essential to ensure uninterrupted supply and regulatory compliance.
In this detailed guide, we will explore Technology transfer in pharma, covering its definition, regulatory expectations, step-by-step process, common challenges, and industry-proven best practices.

What is Technology Transfer in Pharmaceuticals?
Technology transfer in pharma refers to the formal transfer of scientific knowledge, manufacturing processes, analytical methods, and quality documentation from one unit to another. The transferring unit is known as the Sending Unit (SU), while the receiving unit is called the Receiving Unit (RU).
The goal of Technology transfer in pharma is to ensure that the receiving site can manufacture the product consistently, meeting predefined quality specifications and regulatory standards without compromising patient safety.
Technology transfer is not limited to physical processes. It also involves:
Process understanding
Critical material attributes
Process control strategies
Risk assessments
Regulatory commitments
Why Technology Transfer in Pharma is Critical
The importance of Technology transfer in pharma goes beyond operational convenience. It directly impacts business continuity, regulatory approval, and patient outcomes.
Key Reasons Why Technology Transfer is Essential
Ensures Product Quality and Consistency
A well-executed technology transfer ensures that the product manufactured at the receiving site matches the quality of the original product.Supports Commercial Scale-Up
Moving from laboratory or pilot scale to full commercial production requires structured Technology transfer in pharma.Facilitates Global Manufacturing
Multinational pharmaceutical companies rely on technology transfer to manufacture products at multiple sites.Regulatory Compliance
Health authorities expect documented evidence of successful Technology transfer in pharma before approving commercial production.Reduces Manufacturing Risks
Poor technology transfer can lead to batch failures, deviations, recalls, and regulatory observations.
Regulatory Guidelines for Technology Transfer in Pharma
Regulatory agencies worldwide emphasize a controlled and science-based approach to technology transfer.
Key Regulatory Guidelines
ICH Q8 (Pharmaceutical Development)
ICH Q9 (Quality Risk Management)
ICH Q10 (Pharmaceutical Quality System)
WHO Technical Report Series (TRS 961, Annex 7)
FDA Guidance on Process Validation
These guidelines highlight that Technology transfer in pharma is an integral part of product lifecycle management and knowledge management.
Types of Technology Transfer in Pharma
1. R&D to Manufacturing Transfer
This involves transferring formulation, process parameters, and analytical methods from development to the manufacturing site.
2. Site-to-Site Transfer
Involves moving an established process from one commercial site to another due to capacity expansion, cost optimization, or strategic reasons.
3. Scale-Up Technology Transfer
Transition from laboratory or pilot scale to full-scale production.
4. Transfer to Contract Manufacturing Organizations (CMOs)
Outsourcing production requires extensive documentation and control during Technology transfer in pharma.
Technology Transfer in Pharma: Step-by-Step Process
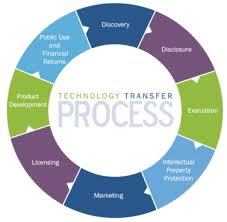
1. Technology Transfer Planning
Planning is the foundation of successful Technology transfer in pharma. A Technology Transfer Plan (TTP) defines:
Scope of transfer
Product and process details
Roles and responsibilities
Timelines and milestones
Risk assessment strategy
Required studies and validation activities
A well-structured plan prevents delays and misunderstandings.
2. Product and Process Knowledge Transfer
This phase focuses on transferring all relevant technical knowledge, including:
Product development reports
Formulation rationale
Process flow diagrams
Critical Quality Attributes (CQAs)
Critical Process Parameters (CPPs)
In-process controls
Knowledge transfer is the backbone of Technology transfer in pharma.
3. Material and Supplier Information Transfer
Raw materials and packaging components significantly impact product quality by the equipment qualification in pharma. During Technology transfer in pharma, the following must be shared:
Approved vendor lists
Material specifications
Supplier qualification data
Change control history
Any material change must be assessed for regulatory impact.
4. Analytical Method Transfer
Analytical methods must perform consistently at the receiving site.
Activities include:
Method transfer protocol preparation
Comparative testing
Method verification or validation
Instrument qualification
Analyst training
Analytical reliability is critical for successful Technology transfer in pharma.
5. Equipment and Facility Assessment
Equipment and facility differences are common challenges in Technology transfer in pharma.
Key activities include:
Equipment comparability studies
Gap analysis
Utilities evaluation (HVAC, water systems)
Environmental condition assessment
If differences exist, scientific justification or process optimization may be required.
6. Engineering and Exhibit Batches
Engineering batches help evaluate process performance at the receiving site before validation.
Benefits include:
Identifying scale-related issues
Optimizing process parameters
Training operators
These batches reduce risk during Technology transfer in pharma.
7. Process Validation and Qualification
Process validation confirms that the transferred process is capable of consistently producing quality products.
Validation activities include:
Process Performance Qualification (PPQ)
Cleaning validation
Hold time studies
Continued Process Verification (CPV)
Validation is a regulatory requirement in Technology transfer in pharma.
8. Training and Competency Building
Human factors play a major role in technology transfer success.
Training should cover:
Manufacturing procedures
Critical process controls
Deviation management
GMP requirements
Well-trained personnel ensure sustainability of Technology transfer in pharma.
9. Technology Transfer Report and Closure
A final technology transfer report summarizes:
Activities performed
Results obtained
Deviations and resolutions
Conclusions and recommendations
Formal closure ensures accountability in Technology transfer in pharma.
Key Challenges in Technology Transfer in Pharma
Despite structured processes, challenges often arise.
1. Incomplete Development Data
Lack of scientific rationale can lead to process failures at scale.
2. Scale-Up Risks
Processes optimized at small scale may behave differently at commercial scale.
3. Equipment Differences
Non-identical equipment can impact mixing, granulation, drying, or compression.
4. Analytical Variability
Differences in instruments and analysts can affect data reliability.
5. Communication Gaps
Poor coordination between sending and receiving units is a major risk in Technology transfer in pharma.
6. Regulatory Risks
Incomplete documentation can result in regulatory queries or delays.
Best Practices for Successful Technology Transfer in Pharma
1. Cross-Functional Collaboration
Involve R&D, manufacturing, QA, QC, engineering, and regulatory teams from the start.
2. Strong Knowledge Management
Capture process understanding, not just documents.
3. Risk-Based Approach
Use tools like FMEA to identify and mitigate risks early in Technology transfer in pharma.
4. Early Manufacturing Involvement
Manufacturing input during development improves transfer success.
5. Standardized Documentation
Use approved templates and SOPs for consistency.
6. Robust Change Control
Manage all changes through formal change control systems.
7. Continuous Communication
Regular review meetings ensure alignment between SU and RU.
Role of Quality Assurance in Technology Transfer
Quality Assurance ensures that Technology transfer in pharma complies with GMP and regulatory expectations.
QA responsibilities include:
Approval of transfer protocols
Review of validation activities
Oversight of deviations and CAPAs
Support during regulatory inspections
QA acts as the guardian of quality throughout the technology transfer lifecycle.
Technology Transfer in Pharma and Product Lifecycle Management
Technology transfer is not a one-time activity. It continues throughout the product lifecycle.
Lifecycle considerations include:
Process optimization
Post-approval changes
Continuous improvement
Knowledge updates
Effective Technology transfer in pharma supports long-term product success.
Digitalization and Technology Transfer
Modern pharmaceutical companies increasingly use digital tools to enhance Technology transfer in pharma, including:
Electronic batch records
Knowledge management systems
Data analytics
Digital validation platforms
Digitalization improves traceability, consistency, and efficiency.
Conclusion
Technology transfer in pharma is a critical, science-driven process that ensures consistent product quality across different manufacturing stages and sites. A well-planned and well-executed technology transfer minimizes risks, ensures regulatory compliance, and supports efficient commercialization.
By following structured processes, addressing challenges proactively, and adopting best practices, pharmaceutical organizations can achieve successful and sustainable Technology transfer in pharma, ultimately ensuring safe and effective medicines reach patients worldwide.
Frequently Asked Questions (FAQs)
1. What is the main objective of technology transfer in pharma?
The main objective of Technology transfer in pharma is to ensure consistent manufacturing of a quality product at the receiving site.
2. Who is responsible for technology transfer?
Technology transfer is a shared responsibility among R&D, manufacturing, QA, QC, engineering, and regulatory teams.
3. Is technology transfer required for regulatory approval?
Yes, regulators expect documented evidence of successful Technology transfer in pharma before commercial production.
4. How long does technology transfer take?
The duration depends on product complexity, scale, and regulatory requirements.
5. What documents are critical in technology transfer?
Technology Transfer Plan, development reports, analytical transfer protocols, validation documents, and final transfer reports are essential in Technology transfer in pharma.