Process validation in pharma is one of the most critical regulatory requirements in pharmaceutical manufacturing. It ensures that a manufacturing process consistently produces products meeting predetermined quality attributes and regulatory standards.
Regulatory agencies such as US FDA, EMA, WHO, and CDSCO require documented evidence that pharmaceutical processes are validated before commercial distribution. Without proper process validation in pharma, companies risk batch failures, regulatory observations, product recalls, and compliance issues.
This detailed guide explains process validation in pharma including its types, lifecycle phases, documentation requirements, regulatory expectations, and practical implementation strategies.
What is Process Validation in Pharma?
Process validation in pharma is a documented program that provides a high degree of assurance that a specific manufacturing process will consistently produce a product meeting its quality specifications and attributes.
It is not a one-time activity. Modern process validation in pharma follows a lifecycle approach that includes process design, qualification, and continued process verification.
The primary objective of process validation in pharma is to:
Ensure consistent product quality
Minimize variability
Reduce batch failures
Maintain regulatory compliance
Improve process understanding
Why Process Validation in Pharma is Important ?
Process validation in pharma is essential because pharmaceutical products directly impact patient safety. Any variation in manufacturing can affect product efficacy and safety.
Key Reasons:
Ensures product consistency
Reduces manufacturing risks
Improves process control
Supports regulatory approvals
Builds scientific process understanding
A properly executed process validation in pharma reduces rework, rejection, and deviation rates significantly.
Regulatory Guidelines for Process Validation in Pharma
Process validation in pharma is guided by:
US FDA Guidance for Industry (Process Validation: General Principles and Practices)
WHO Technical Report Series
EU GMP Annex 15
ICH Q8, Q9, Q10 Guidelines
All these guidelines emphasize lifecycle-based process validation in pharma rather than a one-time validation exercise.
Types of Process Validation in Pharma
Process validation in pharma ensures that manufacturing processes consistently produce products meeting predetermined quality attributes. One of the most important concepts in process validation in pharma is understanding the different types of validation used depending on the stage of manufacturing and regulatory requirements.
Prospective Validation
Prospective validation is the most common and preferred type of process validation in pharma. It is conducted before commercial distribution of a product.
This type of process validation in pharma is performed during product development or before routine production begins. The objective is to demonstrate that the manufacturing process will consistently produce quality products under defined conditions.
When is Prospective Validation Used?
Launch of a new product
New manufacturing process
New manufacturing site
Major formulation change
Technology transfer
Key Features
Conducted prior to commercial release
Based on development and pilot batch data
Usually involves three consecutive successful validation batches
Includes detailed protocol and sampling plan
Example
If a company develops a new tablet formulation, prospective process validation in pharma will be conducted using three commercial-scale batches. Data such as assay, dissolution, content uniformity, and impurities are evaluated to confirm consistency.
Advantages
Regulatory preferred method
Scientifically controlled
Lower risk compared to other types
Prospective validation is considered the gold standard in process validation in pharma.
Concurrent Validation
Concurrent validation is performed during actual production of commercial batches.
In this type of process validation in pharma, the product is manufactured and distributed while validation data is being collected and evaluated.
When is Concurrent Validation Used?
Urgent medical demand
Rare or orphan drugs
Limited batch production
When prospective validation is not practical
Key Features
Conducted during routine production
Requires intensive monitoring and testing
Batch release often depends on validation data review
High level of QA oversight required
Example
If a life-saving drug is urgently required in the market, concurrent process validation in pharma may be performed while supplying the product, provided sufficient justification and regulatory approval exist.
Risks
Higher regulatory scrutiny
Greater risk compared to prospective validation
Concurrent validation must be well-justified and scientifically supported.
Retrospective Validation
Retrospective validation is based on historical batch data of a product that has already been manufactured and distributed.
This type of process validation in pharma analyzes past production records to demonstrate process consistency.
When is Retrospective Validation Used?
Long-standing products with stable processes
When sufficient historical data is available
No recent process changes
Requirements
At least 10–30 consecutive batch records
No significant deviations
Consistent yield and test results
Stable manufacturing conditions
Example
If a product has been manufactured consistently for years without major changes, retrospective process validation in pharma can analyze historical data to confirm stability.
Regulatory Perspective
Modern regulatory agencies rarely accept retrospective validation for new products. It is mostly applicable for legacy products.
Retrospective validation is now less common due to lifecycle-based approaches in process validation in pharma.
Revalidation
Revalidation is performed when changes occur that could impact product quality.
In process validation in pharma, revalidation ensures that modified processes still produce consistent and compliant products.
When is Revalidation Required?
Change in equipment
Change in manufacturing site
Change in batch size
Change in raw material supplier
Significant deviation trends
Process optimization
Regulatory requirement
Types of Revalidation
a) Full Revalidation
Complete validation process repeated.
b) Partial Revalidation
Only affected parameters are revalidated.
Example
If a tablet compression machine is replaced with a new model, revalidation in process validation in pharma is required to ensure that compression force, hardness, and dissolution remain within specifications.
Revalidation ensures continued process reliability.
Comparison of Types of Process Validation in Pharma
| Type | When Performed | Risk Level | Regulatory Preference |
|---|---|---|---|
| Prospective | Before commercialization | Low | Highly Preferred |
| Concurrent | During production | Medium to High | Accepted with justification |
| Retrospective | After production (historical data) | Medium | Rarely preferred now |
| Revalidation | After changes | Depends on change | Mandatory when applicable |
Understanding these differences is essential for proper implementation of process validation in pharma.
Modern Lifecycle Approach
Today, regulatory authorities promote lifecycle-based process validation in pharma, which integrates:
Process Design
Process Qualification
Continued Process Verification
This approach reduces reliance on retrospective validation and emphasizes continuous monitoring.
Key Considerations for Selecting the Right Type
When selecting the type of process validation in pharma, consider:
Stage of product lifecycle
Regulatory expectations
Availability of historical data
Risk assessment results
Nature of process changes
A risk-based approach ensures compliance and efficiency.
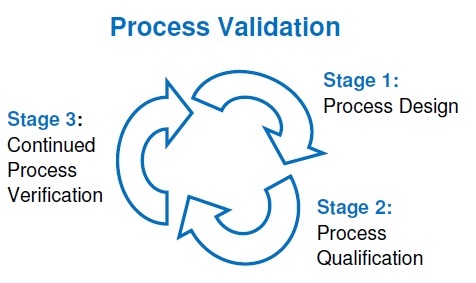
Lifecycle Approach to Process Validation in Pharma
The lifecycle approach to process validation in pharma is a modern regulatory concept introduced by the US FDA in its 2011 guidance titled “Process Validation: General Principles and Practices.” This approach replaced the traditional “three validation batches” concept with a more scientific, risk-based, and continuous monitoring system.
The lifecycle approach ensures that process validation in pharma is not treated as a one-time activity but as an ongoing process that starts from product development and continues throughout the commercial life of the product.
Stage 1: Process Design
Stage 1 is the foundation of the lifecycle approach to process validation in pharma. In this stage, the commercial manufacturing process is defined and developed based on scientific knowledge gained during product development.
The objective of Stage 1 is to design a robust process capable of consistently delivering quality products.
Activities Include:
Identifying Critical Quality Attributes (CQA)
Identifying Critical Process Parameters (CPP)
Risk assessment (FMEA)
Design of Experiments (DoE)
Scale-up studies
Scientific understanding is the foundation of process validation in pharma.
Stage 2: Process Qualification
Stage 2 confirms that the designed process performs effectively in the commercial manufacturing environment. It demonstrates that facilities, equipment, utilities, and processes are capable of reproducible performance.
It has two parts:
Equipment Qualification
IQ (Installation Qualification)
OQ (Operational Qualification)
PQ (Performance Qualification)
Process Performance Qualification (PPQ)
Typically involves 3 consecutive commercial batches demonstrating consistent quality.
Process validation in pharma at this stage proves reproducibility.
Stage 3: Continued Process Verification (CPV)
Stage 3 ensures that the process remains in a state of control throughout the product lifecycle. This stage distinguishes the lifecycle approach from traditional validation.
Process validation in pharma does not end after three batches. Continuous monitoring is required.
Activities Include:
Trend analysis
Statistical monitoring
Annual Product Quality Review (APQR)
Continued stability monitoring
Process validation in pharma is not complete without continued verification.
Key Components of Process Validation in Pharma
Successful process validation in pharma requires:
Defined manufacturing process
Approved master batch record
Qualified equipment
Validated analytical methods
Trained personnel
Statistical data evaluation
Documentation in Process Validation in Pharma
Documentation in process validation in pharma is one of the most critical elements for ensuring regulatory compliance, traceability, and scientific justification of manufacturing consistency. In pharmaceutical manufacturing, if an activity is not documented, it is considered not done. Therefore, documentation in process validation in pharma provides written evidence that the process has been scientifically evaluated, tested, and proven to consistently produce products meeting predetermined quality specifications. Regulatory agencies such as US FDA, EMA, WHO, and CDSCO strictly review validation documents during audits and inspections to confirm compliance with GMP requirements.
Validation Master Plan (VMP)
The VMP provides an overview of validation strategy.
It includes:
Validation policy
Responsibilities
Scope
Schedule
Risk assessment approach
Process Validation Protocol
The protocol defines how process validation in pharma will be executed.
It includes:
Objective
Scope
Batch size
Sampling plan
Acceptance criteria
Statistical tools
Batch Manufacturing Record (BMR)
The Batch Manufacturing Record (BMR) is one of the most important controlled documents in pharmaceutical manufacturing. It provides complete written evidence of how a specific batch of a product was manufactured, processed, and controlled according to approved procedures.
In simple terms, the Batch Manufacturing Record (BMR) is the actual production history of a batch. It ensures traceability, accountability, compliance, and product quality.
Regulatory authorities such as US FDA, WHO, and EU GMP strictly review Batch Manufacturing Records during inspections to verify compliance with Good Manufacturing Practices (GMP).
Validation Report
After execution, a report summarizes:
Results
Deviations
Statistical analysis
Conclusion
The validation report officially approves process validation in pharma.
Statistical Tools Used in Process Validation in Pharma
Statistical analysis strengthens process validation in pharma.
Common tools include:
Process capability (Cp, Cpk)
Control charts
Trend analysis
Standard deviation
Regression analysis
Statistical evaluation confirms process robustness.
Critical Process Parameters (CPP) and Critical Quality Attributes (CQA)
Understanding CPP and CQA is central to process validation in pharma.
CQA:
Attributes that affect product safety and efficacy.
Examples:
Assay
Dissolution
Impurities
Content uniformity
CPP:
Process parameters impacting CQAs.
Examples:
Mixing time
Temperature
Compression force
Drying time
Proper control ensures successful process validation in pharma.
Common Challenges in Process Validation in Pharma
Inadequate development data
Improper risk assessment
Poor documentation practices
Equipment variability
Lack of statistical understanding
Addressing these challenges improves process validation in pharma outcomes.
Best Practices for Process Validation in Pharma
Use risk-based approach
Involve cross-functional teams
Maintain detailed documentation
Apply statistical evaluation
Ensure continuous monitoring
Following best practices ensures regulatory compliance.
When is Revalidation Required?
Revalidation in process validation in pharma is required when:
Significant process changes occur
Equipment replacement
Scale change
Trend failures observed
Regulatory recommendation
Timely revalidation prevents compliance risks.
Difference Between Process Validation and Cleaning Validation
Process validation in pharma ensures consistent product manufacturing.
Cleaning validation ensures removal of residues and cross-contamination.
Both are regulatory requirements but serve different purposes.
Role of QA in Process Validation in Pharma
QA is responsible for:
Reviewing validation protocol
Monitoring validation batches
Approving validation reports
Ensuring regulatory compliance
QA oversight ensures integrity of process validation in pharma.
Conclusion
Process validation in pharma is a scientific and regulatory requirement that ensures manufacturing consistency and patient safety. By following lifecycle-based validation, applying statistical analysis, maintaining proper documentation, and ensuring continuous monitoring, pharmaceutical companies can achieve robust and compliant manufacturing processes.
A well-implemented process validation in pharma not only satisfies regulatory authorities but also improves operational efficiency and product quality.
Frequently Asked Questions (FAQ)
Q1. What is process validation in pharma?
Process validation in pharma is documented evidence that a manufacturing process consistently produces quality products meeting predetermined specifications.
Q2. How many batches are required for process validation in pharma?
Typically, three consecutive successful batches are required for process validation in pharma, though this may vary based on risk assessment.
Q3. What are the stages of process validation in pharma?
The three stages are Process Design, Process Qualification, and Continued Process Verification.
Q4. When is revalidation required in process validation in pharma?
Revalidation is required after process changes, equipment modifications, or trend failures.
Q5. What documents are required for process validation in pharma?
Key documents include Validation Master Plan, Validation Protocol, Batch Records, and Validation Report.