Global regulatory authorities consistently highlight the importance of documentation and data integrity.
Key guidelines include:
Regulators evaluate Good documentation practices in pharma during inspections by reviewing:
Completeness of records
Data consistency and traceability
Corrections and error handling
Electronic system controls
Record retention and archiving
Why Documentation Is Critical in Pharma
Documentation serves multiple critical purposes:
Proof of compliance: Demonstrates adherence to GMP
Traceability: Tracks who did what, when, and why
Consistency: Ensures reproducible processes
Risk management: Helps identify deviations and trends
Legal evidence: Supports regulatory and legal requirements
Weak documentation undermines the entire quality system. That is why Good documentation practices in pharma are considered a foundational GMP requirement.
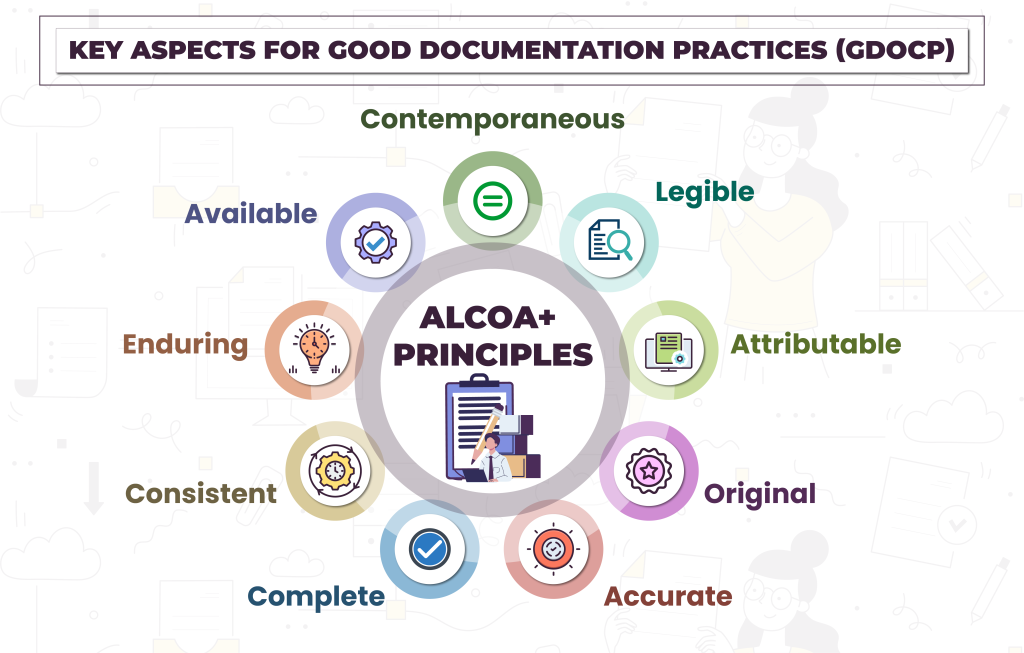
Understanding ALCOA and ALCOA+
ALCOA is a set of principles originally defined to ensure data integrity. Over time, regulators expanded it to ALCOA+ to address modern risks, especially electronic data management.
ALCOA Principles
Attributable
Legible
Contemporaneous
Original
Accurate
ALCOA+ Adds
Complete
Consistent
Enduring
Available
Together, ALCOA+ forms the backbone of Good documentation practices in pharma.
ALCOA+ Explained with Practical GMP Examples
1. Attributable
Every data entry must clearly identify who performed the activity and when.
GMP Example:
Non-compliance example:
Attributability ensures accountability and traceability under Good documentation practices in pharma.
2. Legible
Records must be readable, permanent, and understandable throughout their retention period.
GMP Example:
Non-compliance example:
Illegible data is treated as missing data during audits.
3. Contemporaneous
Data must be recorded at the time the activity is performed, not later.
GMP Example:
Non-compliance example:
Contemporaneous recording is a core expectation of Good documentation practices in pharma.
4. Original
Original records are the first capture of data or a verified true copy.
GMP Example:
Non-compliance example:
5. Accurate
Data must reflect the true value without manipulation or selective reporting.
GMP Example:
Accuracy is essential to maintain trust in Good documentation practices in pharma.
6. Complete
All data, including repeats, rejections, and deviations, must be included.
GMP Example:
Non-compliance example:
7. Consistent
Data should follow a logical sequence with consistent time stamps and formats.
GMP Example:
8. Enduring
Records must be preserved in durable media for the entire retention period.
GMP Example:
9. Available
Records must be readily retrievable for review or inspection.
GMP Example:
Availability is a key inspection focus in Good documentation practices in pharma.

Types of GMP Documents Covered Under GDP
Controlled Documents
SOPs
Policies
Protocols
Specifications
Uncontrolled / Operational Records
BMRs and BPRs
Equipment logbooks
Laboratory worksheets
Both categories must comply with Good documentation practices in pharma.
GDP Rules for Handwritten Entries
Key rules include:
Use permanent ink
No backdating
No correction fluid
Single-line strike-through for corrections
Initial, date, and reason for correction
Correct error handling is frequently reviewed during inspections.
Good Documentation Practices for Electronic Records
Electronic systems must comply with:
21 CFR Part 11
EU GMP Annex 11
Key controls include:
Unique user IDs
Role-based access
Audit trails
Data backups
Electronic compliance is now inseparable from Good documentation practices in pharma.
Common GDP Errors Observed in Audits
Regulators frequently cite:
Backdated entries
Missing signatures
Uncontrolled documents
Incomplete raw data
Poor archival practices
Most data integrity observations stem from GDP failures.
Best Practices to Strengthen GDP Compliance
Regular GDP training
Simple and clear SOPs
Periodic self-inspections
Use of templates and checklists
Digital QMS implementation
A strong GDP culture supports sustainable compliance.
Role of Training in GDP
Training ensures that personnel:
Training effectiveness directly impacts Good documentation practices in pharma.
GDP and Regulatory Inspections
During inspections, auditors assess:
Strong GDP practices reduce inspection risk and build regulatory confidence.
GDP as a Foundation of Quality Culture
Good documentation practices in pharma are not about paperwork—they reflect an organization’s quality mindset. When documentation is accurate, complete, and transparent, it builds trust with regulators and ensures patient safety.
Conclusion
Good documentation is the backbone of pharmaceutical quality systems. By applying ALCOA+ principles consistently, organizations can ensure data integrity, regulatory compliance, and operational excellence. Good documentation practices in pharma protect patients, support inspections, and enable continuous improvement in an increasingly regulated global environment.
FAQ: Good Documentation Practices in Pharma
1. What are Good documentation practices in pharma?
Good documentation practices in pharma are a set of GMP principles that ensure all pharmaceutical records are accurate, complete, traceable, and reliable. They confirm that activities were performed as approved and help maintain data integrity throughout the product lifecycle.
2. Why are Good documentation practices in pharma important?
Good documentation practices in pharma are critical because documentation serves as legal evidence of compliance. Poor documentation can lead to regulatory observations, warning letters, product recalls, and loss of market authorization.
3. What does ALCOA+ mean in GDP?
ALCOA+ represents data integrity principles used in Good documentation practices in pharma:
Attributable
Legible
Contemporaneous
Original
Accurate
Complete
Consistent
Enduring
Available
These principles ensure data reliability in both paper and electronic records.
4. Is ALCOA+ mandatory under GMP?
Yes. While ALCOA+ may not always be written word-for-word in regulations, global regulators expect compliance with these principles as part of Good documentation practices in pharma and data integrity requirements.
5. Which documents are covered under GDP?
GDP applies to all GMP-related documents, including SOPs, batch manufacturing records, analytical worksheets, logbooks, validation documents, calibration records, training records, and electronic data.
6. How should errors be corrected in GMP documents?
Errors must be corrected using a single-line strike-through, with the correct entry written nearby, along with initials, date, and reason if required. Correction fluid, overwriting, or backdating is not allowed under Good documentation practices in pharma.