In today’s pharmaceutical world, Validation Planning is the backbone of regulatory compliance, patient safety, and product quality. Without a well-structured validation strategy, pharmaceutical manufacturers risk product recalls, audit failures, and loss of credibility.
This article is a comprehensive 3000-word guide on Validation Planning, covering its meaning, objectives, scope, workflow, regulatory requirements, industry examples, best practices, and future trends. Whether you’re a student, professional, or quality leader, this resource will help you master one of the most critical aspects of the pharmaceutical validation framework.
Related: pharmaceutical vessels

What is Validation Planning?
Validation Planning is the process of organizing and documenting all qualification and validation activities in a pharmaceutical facility. It includes defining the scope, responsibilities, validation strategy, timelines, and documentation requirements to ensure equipment, systems, utilities, and processes consistently deliver reliable results.
Instead of treating validation as a one-time task, a master validation plan (VMP) provides a structured, lifecycle-based approach. This ensures compliance with GMP guidelines from the FDA, EMA, WHO, and ICH.
Simply put, Validation Planning ensures that every product manufactured is safe, effective, and consistent — protecting patients and ensuring regulatory trust.
Why is Validation Planning Important?
The pharmaceutical industry operates under strict quality expectations. A strong validation framework brings multiple benefits:
Regulatory Compliance – Demonstrates adherence to GMP and global regulations.
Risk Control – Reduces the risk of contamination, equipment failure, and process deviations.
Audit Readiness – Provides inspectors with a clear roadmap of validation activities.
Consistency in Production – Ensures reproducibility in batch manufacturing.
Cost Efficiency – Prevents rework, rejects, and costly product recalls.
Continuous Improvement – Supports long-term quality system sustainability.
Objectives of Validation Planning
The main goals of Validation Planning are:
Define the scope of validation (systems, utilities, processes, cleaning, computerized systems).
Establish responsibilities across QA, QC, Production, and Engineering.
Apply a risk-based validation approach to prioritize critical systems.
Prepare a structured validation schedule with clear timelines.
Ensure robust documentation (protocols, reports, approvals).
Outline revalidation criteria to address system or process changes.
Maintain an audit-ready validation program at all times.
Components of a Master Validation Plan
A complete Validation Master Plan (VMP) generally contains:
Introduction & Scope – Defines systems and processes covered.
Validation Policy – States the company’s overall validation philosophy.
Roles & Responsibilities – Clear division between QA, QC, Production, and Engineering.
Validation Strategy – Prospective, concurrent, or retrospective validation.
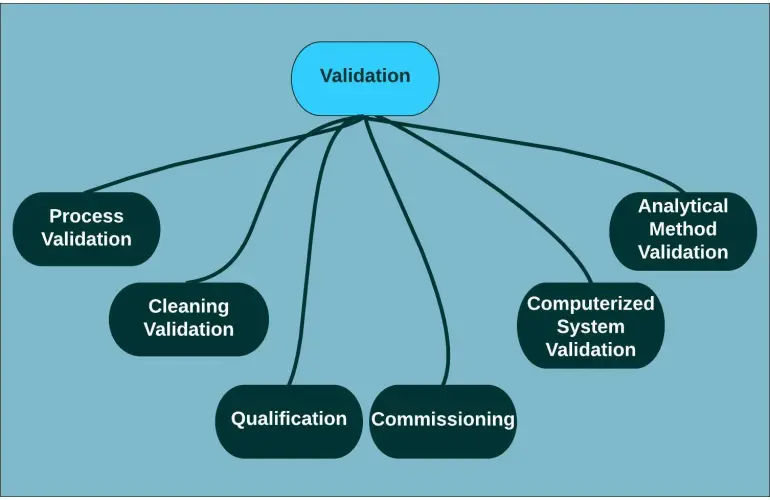
Types of Validation – Process, cleaning, analytical, computer system, equipment qualification.
List of Critical Equipment/Systems – HVAC, water systems, reactors, granulators.
Risk Assessment Framework – Prioritizing validation activities by impact.
Documentation Requirements – Protocols (IQ, OQ, PQ), reports, summary documents.
Change Control & Revalidation – Triggers for repeat validation.
Validation Schedule – Gantt charts or milestone timelines.
Workflow of Validation Planning
A successful validation process follows these steps:
System Identification – Identify equipment, utilities, and processes.
Risk Assessment – Classify systems as critical, major, or non-critical.
Preparation of VMP – Develop a structured plan with scope and timelines.
Protocol Development – Prepare IQ, OQ, PQ, and other protocols.
Execution of Validation – Perform qualification and validation as per protocols.
Report Preparation – Document results, deviations, and conclusions.
Ongoing Monitoring & Revalidation – Establish periodic review and requalification triggers.
Types of Validation in Pharma
In pharmaceutical validation, multiple types are included under Validation Planning:
Process Validation – Ensures processes consistently produce quality products.
Cleaning Validation – Verifies cleaning procedures prevent cross-contamination.
Analytical Method Validation – Confirms reliability of test methods (assay, impurities).
Computer System Validation (CSV) – Ensures software and systems function as intended.
Equipment & Utility Qualification – Includes IQ, OQ, PQ to confirm proper installation and operation.
Validation Planning in API Manufacturing
Example: In an API facility, Validation Planning ensures:
HVAC qualification maintains temperature and pressure differentials.
Water systems (Purified Water, WFI) meet microbial and chemical limits.
Process validation ensures crystallization steps are reproducible.
Cleaning validation prevents cross-contamination between APIs.
Analytical validation confirms reliability of HPLC assay methods.
This ensures compliance with GMP and consistent API quality.
👉 Would you also like me to include a formulation industry example (e.g., tablets, injectables)?
Regulatory Expectations
Different regulatory agencies have strict requirements for validation strategy:
US FDA – Lifecycle approach (Stage 1: Process Design, Stage 2: Process Qualification, Stage 3: Continued Process Verification).
EU EMA (Annex 15) – Requires a comprehensive VMP.
WHO GMP (TRS 937 Annex 4) – Emphasizes risk-based validation.
ICH Q8, Q9, Q10 – Focus on Quality by Design, Risk Management, and Pharmaceutical Quality Systems.
A robust validation framework must address all of these expectations.
Best Practices
Adopt a Risk-Based Validation Approach using tools like FMEA.
Maintain Document Control – Ensure protocols and reports are updated and version-controlled.
Cross-Functional Collaboration – Involve QA, QC, Engineering, Production.
Trigger-Based Revalidation – Perform revalidation after significant changes.
Employee Training – Build competency in validation concepts.
Integration with QMS – Link validation with deviations, CAPAs, and change controls.
Common Challenges
Some frequent obstacles in Validation Planning are:
Poor communication between departments.
Weak documentation practices.
Over-validation or under-validation due to poor risk analysis.
Outdated Validation Master Plans.
Limited resources leading to delays in execution.
Case Study: Failure in Validation Strategy
A mid-sized company received an FDA Warning Letter for failing to revalidate after modifying a granulation process. The lack of a proper validation framework led to inconsistent dissolution results.
📌 Lesson Learned: Always include revalidation triggers in your Master Validation Plan.
Future of Validation Planning
With Pharma 4.0, Validation Planning is evolving:
Digital Validation Management Systems (DVMS) for paperless compliance.
Real-time monitoring during continued process verification.
AI-driven risk analysis for smarter validation prioritization.
Cloud-based validation platforms for global companies.
Read more: validation planning
Conclusion
Validation Planning is not just about compliance — it is about ensuring product quality, patient safety, and operational excellence. A well-designed validation strategy integrates process validation, cleaning validation, equipment qualification, and risk management into a unified framework.
For pharma professionals, mastering Validation Planning means being ready for audits, reducing risks, and building a sustainable quality system. For organizations, it means regulatory trust and business continuity.
Key Takeaways
Validation Planning = Compliance + Quality + Risk Control
A strong Validation Master Plan is essential for every pharmaceutical company.
Future validation will rely on digital systems, AI, and continuous verification.
Frequently Asked Questions (FAQ) on Validation Planning
1. What is Validation Planning in pharma?
Validation Planning is the structured process of preparing, organizing, and documenting all qualification and validation activities in pharmaceutical manufacturing to ensure GMP compliance.
2. Why is Validation Planning important?
It ensures consistent product quality, patient safety, regulatory compliance, risk reduction, and smooth audit readiness.
3. What does a Validation Master Plan include?
A VMP includes scope, responsibilities, validation strategy, risk assessment, documentation requirements, revalidation triggers, and validation schedules.
4. What types of validation are covered?
It includes process validation, cleaning validation, analytical method validation, computer system validation, and equipment qualification (IQ, OQ, PQ).
5. Who is responsible for Validation Planning?
Quality Assurance leads the process, with support from Production, QC, and Engineering teams.